5/20 Comets, Asteroids, Meteoriods.
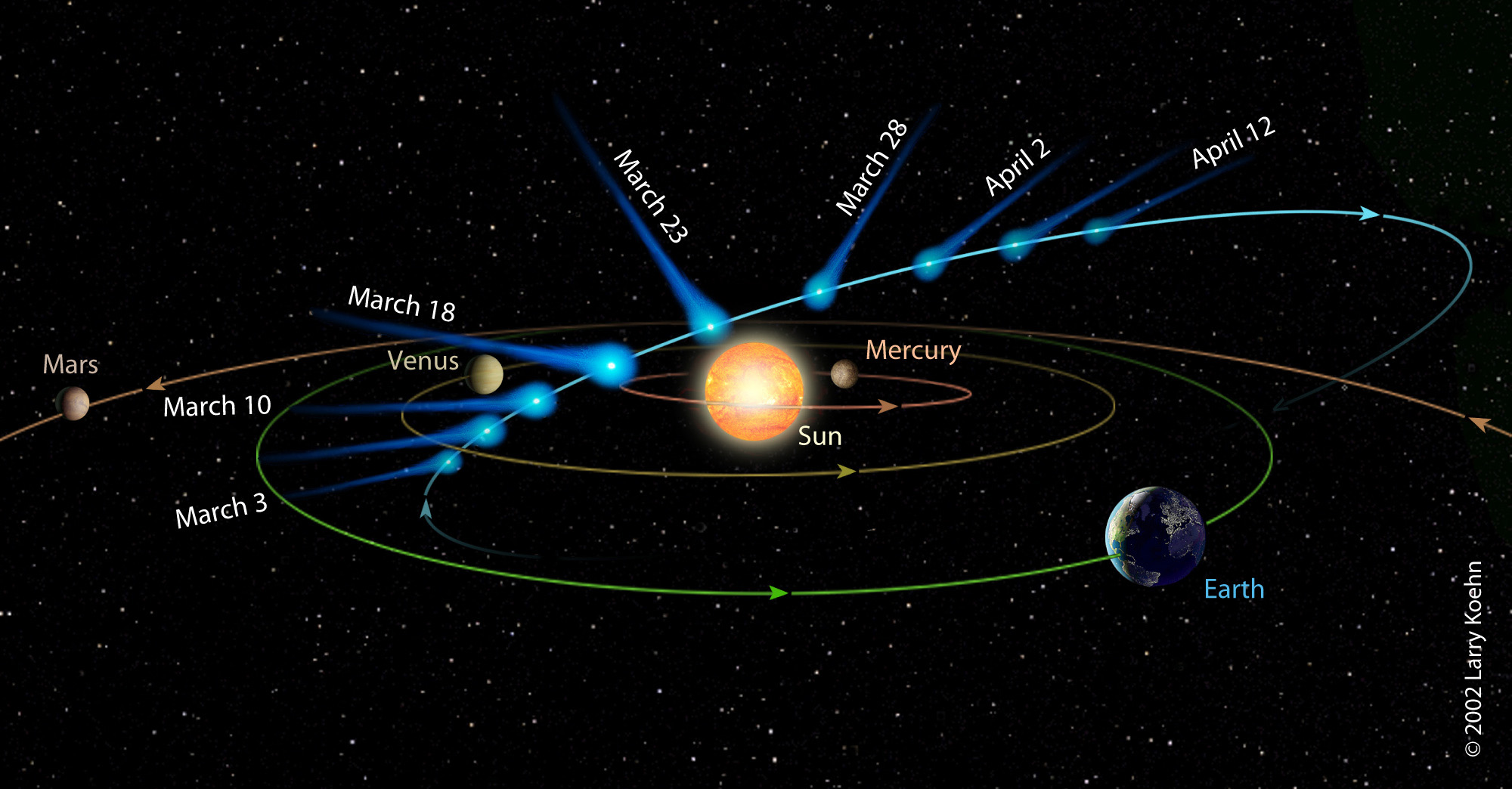
Comet Animation

Asteriod Belt between Mars and Jupiter.

5/19 Criteria for Planets- there are three criteria for planets
1) the object must orbit the sun, 2) must have sufficient mass for gravity to make it into a spherical shape and 3) must have cleared the neighborhood around it's orbit.

5/18 The Sun. the sun is different from all other objects in our solar system. For starters, it's larger than any other object. About 1.3 million Earths could fit inside the sun! Unlike the Earth, the sun is a giant gas sphere of plasma..whereas the Earth is a rocky planet. The tremendous pressure and temperatures on the sun are causing nuclear fusion taking place where hydrogen atoms are fusing together to form helium atoms, and the chemcial reaction is extremely exothermic--giving off enormous amounts of energy. The Sun is at the center of the solar system with all the planets revolving around it.
5/7 The Moon Formation

5/6 Rollercoaster review. Drop Zone Stunt Tower Physics question: Does the weight of the passengers affect how fast the drop zone falls?

Answer: No. Whether you are big or small, fat or thin...gravity affects all objects equally. Don't be fooled into thinking that just because something is larger or heavier, that they fall faster than smaller objects. The only thing that causes some objects to fall slower is air resistance (fluid friction) or aerodynamic drag.
Next question: Does a rollercoaster go faster with or without passengers?

Answer: No. Since a rollercoaster uses gravity to convert it's stored potential energy into speed energy..it's like a fallinlg object..and gravity affects all objects equally. A rollercoaster full of passengers will have more inertia and momentum but that won't affect how fast it will go.
5/5 Tides on Earth There are two high tides and two low tides every day (24 hrs). The moon's gravity is primarily responsible for the high/low tides with the Sun's gravity being secondary. Even though the Sun is much more massive that our Moon, the Moon is much closer by comparison, so we feel the effects of it's gravity more on Earth. The ocean closest to the moon bulges outward causing a high tide, but there is also a high tide on the opposite side of the Earth which is caused by the moon tugging on the Earth and actually pulling the Earth toward the moon by a couple feet which causes some water to be left behind. (see small picture below.


Spring tides are especially high tides that occur twice a month on the New Moon and Full Moon. In this case the Moon and Sun are all aligned in a straight line which increase the gravitational pull on the oceans resulting in higher than normal tides. Neap tides also occur twice a month, when the moon is at first quarter and 3rd quarter moon. This is when the moon and sun form 90 degree angles to one another so that their gravitational forces are cancelling one another..resulting in smaller than normal tides (high and low).

5/4 Solar Eclipse and Lunar Eclipse
A solar eclipse occuring on Earth as view from the sun. (The Moon comes between the Earth and the sun)

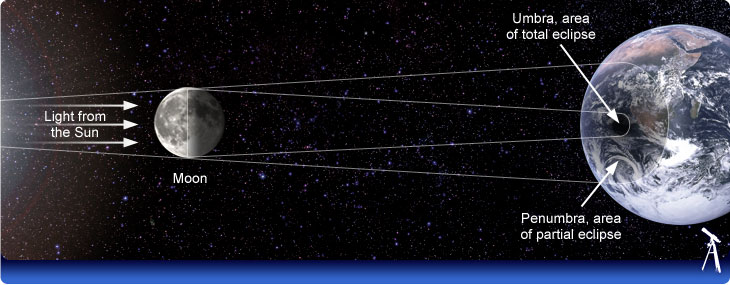
Solar Eclipse as view from the Earth.


Lunar Eclipse. The moon passes into the Earth's shadow.

5/1 Phases of the Moon. If you ever watch how the moon tracks across the sky (whether during the day or night) it follows the sun..in other words it rises in the east and sets in the west (due to the Earth's rotation). The phases we see from Earth are determined solely by the position of the Moon in it's 28 day revolution around the Earth..and how sunlight is being reflected.


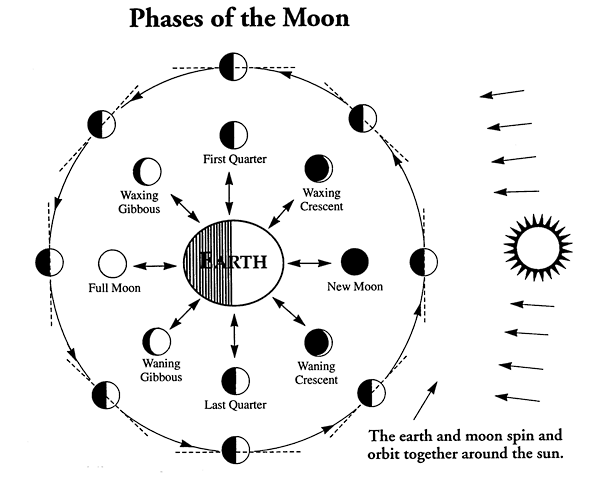
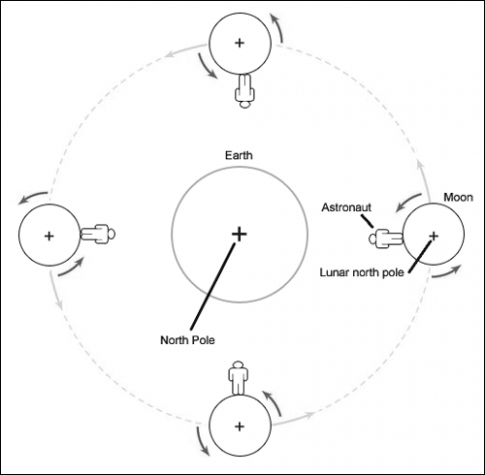
4/30 Moon's Rotation and Revolution is the same ...approx 28 days.This means is that one side of the moon is always facing the Earth. In other words, we've never seen the other side of the moon since the same side is always facing the Earth. (well of course some of the Apollo astronaunts have seen it since they orbited the moon before landing on it). The astronaunts discovered that the other side of the moon which is always facing away from the Earth has more impact craters from meteors/asteroids.
4/29 Tropic of Cancer & Capricorn. The tropic of Cancer is 23.5 degrees of latitude north of the equator. This is the farthest north the sun travels north of the equator, and it reaches this point on the summer solstice. The tropic of Capricorn is 23.5 degrees of latitude south of the equator. This is the farthest south the sun travels in the winter, and it reaches this point on the winter solstice. Notice that the tilt of the Earth is also 23.5 degrees.

4/28 Solstice and Equinox review.


4/27 Stomp Rocket Launch.
4/24 Stomp rocket contest (post poned to Monday) Science World Magazine
4/23 Stomp Rockets and Newton's Laws of Motion.
The more mass the rocket has, the more inertia it will have...so while it may require more force to get it moving, once it gets moving..it'll want to keep moving. The more force you can apply to the bottle, the greater the acceleration of the rocket..so you'd want the person who weighs the most to jump on the bottle. The action of the air coming out of the launcher is causing the rocket to exert an equal and opposite force in the opposite direction which propells the rocket.

4/22 Stomp Rockets Desgin contest. Three factors must be considered when designing your stomp rocket. Rocket (fuselage) lenght can either be short/medium or long). The longer fuselage adds some lift but increases mass. Nose cone mass is necessary to make the rocket fly in the desired direction. Launch angle depends on the wind conditions, with 45 being optimum for no wind. You must determine the best rocket design. Each rocket must have a nose cone and at least two fins.

4/21 Myth Busters Buoyancy video
4/20 Density & Buoyancy How does a submarine sink/dive or rise to the surface? The submarine has some compartment inside the submarine which it allows water to fill in order to increase it's density so that it sinks..When it wants to surface, it forces compressed air into those compartments which pushes out the water and which makes the submarine's density less than water..so it rises to the surface. When a submarine dives, it reduces its buoyant force by taking water into its flotation tanks. Once a submarine is down, it rises again by pushing waterback out. The water is pushed out by filling the flotation tanks with air.

4/17 Graduation Pictures
4/16 Chapter 10 Test
4/15 Standards Review
4/14 Exploratorium Film festival & review
4/13 Geosynchronus orbits and GPS
Satelites in geosynchronus orbits are usually orbiting around 22,000-26,000 miles above the Earth's equator, taking 24 hours to orbit the Earth. Since the time to make a complete orbit equals the rotation of the Earth (24 hrs) the satelite stays above the same position on the Earth, hence the name geo(earth)-synchronus orbit. Many communication and TV satelites maintain these orbits and which is why if you have DISH tv you point your dish towards the south where the DISH tv satelite is in a geosynchronus orbit above the equator.

Global Positioning Satelites (GPS) only orbit about 1/2 as high up (around 12,000 miles) and take about 12 hours to complete a rotation around the Earth (making two complete rotations in a 24 hr period). Because of their altitude above the Earth, there are always at least 4 GPS satilites within range of any point on the Earth. There are 24 GPS in Earth orbit and your car's GPS needs to communicate with at least 3 to triangulate it's position accurately.

3/31 Momentum (P)



3/27 Satellite Motion
There is a huge misconception that if something is in space then there is no gravity acting on it. The universal law of gravity tells us that gravity acts on all objects in the universe. Then why it appear that the space shuttle astronaunts are floating in space when the space shuttle is orbiting earth. First of all, the space shutte orbits the Earth at approximately 150 miles above the surface. At that altitude, there is no atmosphere (so the space shuttle is in space) however the Earth's gravity still exerts a VERY strong pull on the space shuttle. Remember, the Earth's gravitational pull keeps the Moon in orbit. What is happening is in fact the space shuttle is traveling so fast (approx 17,500 mph) ..faster than a speeding bullet, that as the Earth's gravity is pulling the shuttle down to the Earth's surface, the surface is curving away at the same rate..so the astronaunts are actually falling all the time..which makes it look like they are floating. This sensation of falling or weightlessnesscan be uncomfortabe for astronaunts and is often called "space sickness".


3/26 Chap 10 Review & worksheet
3/25 Friction Link to Friction website


3/24 Balanced and Unbalanced Forces


The NET force in the above illustration is 50 Newtons. In the top part of the drawing it's 50 N to the right, and in the bottom part of the drawing, it's 50 N to the left.

3/23 Force

3/20 Work = Potential Energy

3/19 Law of Conservation of Energy

3/18 Extra Credit Parachute Contest

3/17 Kinetic and Potential Energy.


3/13 Parachute Design Considerations.

3/12 Egg Drop Contest

3/11 Egg Drop Activity
3/10 Egg Drop Acitivity. Graphing Acceleration.
Line AB represents acceleration (speed is increasing over time)
Line BC represents no acceleration (speed is constant)
Line CD represents deceleration (speed is decreasing over time)

3/9 Gravity and Freefall Acceleration Click here to learn more about gravity


3/6 Velocity and Acceleration review.
3/5 Field Trip to the Exploratorium. Exploratorium project due March 31, 2009.
3/4 Youth Risk Behavior Survey.
3/3 Inertia. Inertia is an object's resistance to change whatever it is doing. If it is not moving, it wants to remain that way. If it's moving, it wants to keep moving. The more mass an object has, the more inertia it has (the more resistance to change). Slime Activity using polyvinyl alcohol.
3/2 Acceleration. Acceleration is a change in velocity. Remember that velocity is speed plus a direction. So a change in velocity can be either a change in speed or a change in direction.

2/26 Speed Review and Speed Quiz
2/25 Solving Speed Word Problems
2/24 Graphing Speed

2/23 Motion, Reference points and speed.

2/19 Polymers

2/18 Unsaturated Hydrocarbons.

2/17 Hydrocarbons

2/13 Guest Speaker: "Making informed decisions"
2/12 Guest Speaker: "Birth Control Methods"
2/11 Guest Speaker: "Sexually Transmitted Infections"
2/10 Guest Speaker: "Sex or Abstinence?"
2/9 Guest Speaker: "Human Reproductive Anatomy"


2/6 Hydrocarbons: Hydrocarbons are organic compounds made of carbon and hydrogen atoms. There are two basic types: Saturated and Unsaturated. Saturated hydrocarbons have single covalent bonds between all of the carbon atoms. (See Ethane below). Unsaturated hydrocarbons have double (Ethene) or triple (Ethyne) covalent bonds between their carbon atoms.

2/5 Standard Review (Reactions)
2/4 Stop Action Movie Festival!
2/3 Forms of Carbon. There are three main forms of pure carbon. Graphite, Diamond and Fullerenes.

2/2 Chap 8.1 Carbon the Element of LIfe. Carbon easily bonds (covalently) in a variety of ways with itself and other elements. It is the most abundant element on Earth, with more atoms than any other element. All life forms, whether plant or animal require carbon, hence the term "carbon based lifeforms".
1/29 Chapter 7 Assessment
1/28 Unit review
1/27 Change in phase lab. Complete your data table and graph in your journal.
1/22 Stop Action Movie project in computer lab making titles, credits, special effects.
1/21 Stop Action Movie project in computer lab using Moviemaker software. Moviemaker handout (pc)
iMovie handout (mac)
1/16 Antacid Lab Complete Antacid lab investigation in class.
click here for: AntAcid Lab handout for journal.
1/15 Antacid Lab Introduction. Complete Lab Report in Science journal, parts 1) Purpose 2) Materials 3) Procedure in class before Friday.
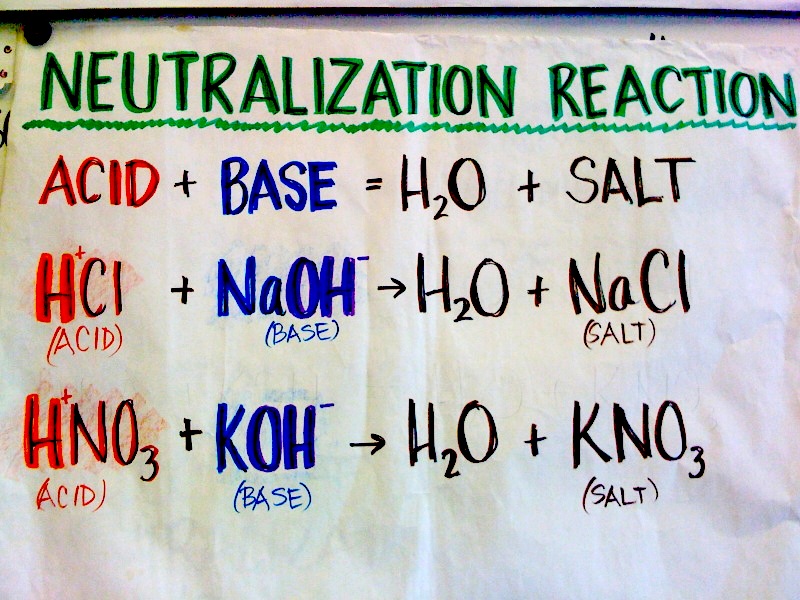
1/14 Neutralization Reactions.

Some examples of Neutralization Reactions.
1/13 ph scale. Methane Candle Lab. Put the lab writeup in your science journal.

1/12 pH scale
The pH scale gets it name from the "power or percent of Hydrogen", referring to the concentration of hydrogen ions. A pH of 7 is considered neutral (neither an acid or a base).

1/7- 1/9 Chap 7.3 Acids & Bases
Acids Bases
Taste Sour & react with metals Taste Bitter & feel slippery
Turn Litmus paper Red Turn Litmus paper Blue
Have a pH less than 7 Have a pH greater than 7
Release H+ ions in water Release OH- ions in water

1/6 Chap 7.2 Solubility. Solubility is the ability of the solvent to dissolve the solute. The type of solvent, temperature and pressure can affect solubility. Some types of solvents and solutes are not compatible ("like dissove like")-for example vinegar (polar) and oil (nonpolar) are not compatible and will not mix or dissolve. Usually as the temperature of the solvent increases, so does it's ability to dissovle more solute (the exception is when the gas is the solute-for example a warm carbonated soda won't hold as much carbonated gas as a cold one). With gases, as you increase the pressure, the more gas (solute) you can dissolve into the solvent.

. If you'd like to make some rock candy for extra credit, see Extra Credit Assignments.

1/5 Chap 7.1 Solutions. Solutions are uniform mixtures that contain a solvent (largest) and at least one solute (smaller). Some general characteristics of solutions: they can be made with or without water, they can be solids, liquids or gases, they are physical changes (you can let saltwater evaporate, and the salt is left behind). Solutes usually lower the freezing point (adding salt to water lowers the freezing or melting point which is why they add salt to icy roads in the winter, once the ice melts it becomes saltwater and can't refreeze). Solutes usually raise the boiling point (adding salt to water to make pasta increases the temperature of the boiling water so the pasta cooks faster as well as making it taste better). Link about Solutions (click here), Link to Mixtures, More Mixtures, Mixture examples.
12/18 Chap 6 Test
12/17 Chap 6 review/ Stop Action Movie project
12/16 Chap 6 Chemical Reactions review
12/15 Periodic Table of Elements Pictionary Game/PPt
Fire Triangle is made up of fuel, temperature, and oxygen.

12/12 Structure of Matter Standards Review Continued. Go over answers, answer questions.
12/11 Structure of Matter Standards Review pgs. 41-61 in Standards Review Workbook.
12/10 Video on Chemical Bonds, Types of Reactions, Factors affecting rates. Followed by video quiz.
12/9 Egg in a bottle activity (youtube video)
12/9 Chapter 6.3 Factors Affecting Rates of Chemical Reactions.

12/8 Classifying Chemical Reactions.

12/5 Exothermic/Endothermic Investigation link to exo/endo lab
12/4 Chemical Bonds (Ionic/Covalent) Review Anytime bonds are broken and/or new bonds are formed then there is a chemical change. If bonds are not being broken and/or new ones formed, then there is no chemical change. See if you can correctly answer this question: When you hold an ice cube in your hand it feels really cold and starts melting. Is this an endothermic reaction? (is there a chemical change or just a change is state (physical change)? It's not endothermic, it's just melting! It's still H2O before and after.
12/3 Chap 6.1 Chemical Change. The four things that are usually considered evidence of a chemical change are 1) production of a gas 2) formation of a precipitate 3) change is color 4) heat given off or absorbed. During a chemical change, bonds are being broken or formed (electrons are being transferred, shared etc) which creates new molecules or compounds. A chemical reaction (when bonds are being broken or formed) is either Exothermic or Endothermic. Exothermic reactions are when energy is given off in the form of heat, light or sound. Endothermic reactions are when the heat is absorbed (cold).
12/2 Chapter 5 Test.
12/1 Chemical Bonding Review. To watch the short movie on bonding click here.
11/25 Molecule Mobile turn in.

11/24 Molecule Mobile In class work
11/21 Molecule Mobile In class work.
11/20 Teen Species video.
11/19 Molecule Mobile In class work.
11/18 Metallic Bonds This last type of bonding is with metals. Since most metals have a valence of 1 to 3 electrons, they easily give them up resulting in a positive metal ion surrounded by a "sea" of electrons which are free to move from atom to atom. These free electrons make metals good thermal (heat) and electrical conductors. They are malleable which means they can be rolled out into sheets (like aluminum foil), they're also ductile which means they can be bent easily or pulled into wires. They also have luster.

Metallic Bonds are good conductors of electricity and thermal (heat) energy. They are malleable which means they can be flattened and rolled into sheets like aluminum foil. They are also ductile, meaning they can be bent easily and pulled into wires, as well as having luster (shiny).
11/17 Molecule Mobile Group Project. Your group must creat a 3-D molecule mobile consisting of at least 4 molecules (minimum 1 molecule per member). Two Ionic and Two Covalent (1 poloar, 1 non polar). Your group must use the guidelines discussed in class: Colors and Size of atoms must be consistent throughout the mobile.

11/13 Polar and Non Polar Covalent Bonds. When some covalent molecules share electrons..one atom may hog or exert a stronger pull on the electrons being shared (this is known as electronegativity). In the water molecule, the oxygen atom exerts a stronger pull on the electrons being shared than the hydrogen atoms..so the electrons tend to gather or hang out around the oxygen atom, giving it a "negative" charge..whereas the hydrogen atoms take on a "positive" charge..(see diagram below).

Carbon dioxide is an example of a nonpolar covalent molecule.

11/12 Covalent Bonding covalent bonding is when atoms "share" their valence electrons to get 8 electrons in the outer shell. In the example below, hydrogen only has one electron, but would like to have two, so it shares it's one electron with oxygen, and in return oxygen shares one of it's 6 valence electrons with hydrogen. So in the water molecule below, they hydrogen atoms now have 2 electrons each in the only shell, and the oxygen has 8 electrons. Everyone is happy!

for a video on covalent bonding: www.youtube.com/watch
11/7 Ionic Bonding Review / Pop Quiz / Stamping Journals
11/6 Goo Activity
11/5 Ionic Bonding - Atoms that lose or gain electrons to get 8 electrons in the last shell are ionically bonded. When an atom like Sodium (Na) gives up it's one electron to Chlorine (Cl)..it become a positive ion, and chlorine becomes a negative ion. Opposites charges (ions) attract!!

For more information about Ionic Bonding: www.chem4kids.com/files/atom_bonds.html
For a video clip about ionic bonding: www.youtube.com/watch
11/4 - Chap 5 Lewis Dot Diagrams. The dots around the element symbol represent the atom's valence (number of electrons in the last shell).

11/3 - Chap 4 (Periodic Table) Test
BOO! (Happy Halloween!) 10/31 - Hydrogen vs Helium Balloons!. The Germans were the first to build airships like the Hindenburg filled with hydrogen gas. Hydrogen is lighter (less dense that helium because it only has an atomic mass of 1 where as helium has an atomic mass of 4. Hydrogen has one very big disadvantage over helium..and if you look at the periodic table you'll understand why. Hydrogen is located on the left side (making it very reactive) and helium is located on the far right side of the periodic table making it non reactive. Watch the slow motion youtube video of a hydrogen balloon being set on fire. www.youtube.com/watch
For a classroom demonstration: www.youtube.com/watch
An even more violent explosion is when both hydrogen and oxygen gas are put in the balloon. www.youtube.com/watch For a classroom demonstration: www.youtube.com/watch
10/30 - Radioactivity. Some atoms have too many (positive) protons in their nucleus (which are repelling each other) that they become unstable..and some subatomic particles are ejected from the nucleus which we call "radioacitivity". There are basically 3 different types of radioacitivity. 1) Alpha decay is when a alpha particle (2protons&2neutrons) is ejected from the nucleus. An alpha particle is basically a helium atom without it's electrons. When an alpha particle is ejected, the nucleus' atomic number decreases by 2, and it's atomic mass/weight decreases by 4.

2) Radioactive Beta Decay is when an atom emits a beta particle (electron). This usually happens when one of the neutrons morphs (changes) into a protron and emits a electron. This results in the atomic number increasing by 1 which changes the atom into a completely different atom. Take the isotope, Carbon-14 which has 6 protons and 8 neutrons (6+8=14). When Carbon-14 undergoes radioactive decay, one of the neutrons changes into a proton (6+1=7) changing carbon into Nitrogen-14!!!

3) Gama radiation. A lot of times when an atom undergoes beta decay (neutron changing into a proton) along with an electron being emitted, a high energy photon is also emitted. In the example below, cobalt-60 undergoes beta decay to become nickle-60 but is too exicted and in order to drop down to a lower energy state, it emits a high energy photon known as gamma radiation. Think of it this way..when you get really, really excited..you like to scream (sound energy)..and since an atom can't scream when it gets excited, it gives off a burst of electromagnetic energy..

Alpha particles can be stopped by a piece of paper, beta particles by wood, and only concrete or lead will stop gama rays.

For more information on radioactivity, see link: www.chem4kids.com/files/atom_nucleus.html
For a goofy song about radioactive decay see: www.youtube.com/watch
10/29 - Noble Gases and Transition Metals. The noble gases are the family of elements on the far right side of the periodic table, and they all have a valence of 8, which means they're "happy" and won't react with any other elements.

For more information on the Noble (Inert) Gases, click on the link: www.chem4kids.com/files/elem_inertgas.html
The transition metals are the largest family and they usually have a valence of 1 or 2. Unlike the all the other families, the transition metals use electrons from their last two shells to bond/react with other elements.

For more detailed information about the transition metals, click on the link: www.chem4kids.com/files/elem_transmetal.html
10/28 - Halogen Family. All the elements that make up the Halogens have a valence of 7. Since they only need one electron to be happy, the Halogens easily react with most elements to gain an electron rather than share one making them a negative ion.

To learn more about the Halogens, click on the link: www.chem4kids.com/files/elem_halogen.html
Oxygen Family. All of the elements that make up the Oxygen Family have a valence of 6. They react with other elements by gaining or sharing 2 electrons in order to bring their valence to 8 (Octet rule).

To learn more about the oxygen family click on the link: www.learner.org/interactives/periodic/groups6.html
10/27 - Nitrogen Family. All of the elements that make up the Nitrogen Family have a valence of 5. They all react with other elements by gaining (or sharing) 3 electrons in order to bring the valence of it last shell to 8.

10/24 - Carbon Family. All of the elements that make up the Carbon Family have a valence of 4. All the elements of this family can form 4 bonds, the most of any element. Carbon is one of the most important elements on the periodic table since it is found in all living things (plants & animals)
. 
See the following link for more detailed information: www.learner.org/interactives/periodic/groups8.html
10/23 - Boron Family. All of the elements that make up the Boron Family have a valence of 3, they don't react with water, oxidize quickly, and react with Halogens or Hydrogen to form ionic salts such as AlH3 or GaCl3

To learn more about the Boron family click on the link: www.learner.org/interactives/periodic/groups9.html
10/22 - Opera Ala Carte (La Boheme)/Correcting quiz and homework. Work on 3-D Atom project.
10/21 - Alkaline Earth Metals. The Alkaline Earth metals are the second most reactive elements on the periodic table..next to the alkali metals. They all have a valence of 2, and so easily give up their two electrons so that they'll drop to the next lower shell which has 8!!! They got their name as the Alkaline Earth metals because when these metals are mixed in solutions they usually form solutions that have a pH greater than 7 making them bases or alkaline. If you have aquariums at home and test the water, you know that the water can either be acidic or alkaline (basic).

For more detailed information click on the link: www.chem4kids.com/files/elem_alkalineearth.html
10/20 - Alkali Metals. The alkali metals are the most reactive family of elements on the periodic table. They all have a valence of 1 (1 electron in their last shell), but the next shell has 8!!!! Remember the octet rule, well, it's much easier for the alkali metals to give up this one electron (rather than try to steal or borrow 7 electrons)..and so once they lose that one electron in their last shell, their next shell now has 8! The Alkali Metals are the most reactive elements on the periodic table. If you want to see just how reactive, clink on the link to watch an amazing YouTube video: (thanks to Christopher Werby for this link!) http://www.youtube.com/watch?v=m55kgyApYrY

See the following link for a more indepth explanation of Alkali Metals. www.chem4kids.com/files/elem_alkalimetal.html
10/17 - Stamping science journals. Collecting Homework. Quiz on Bohr Model.
10/16 - Collect and correct Bohr Model worksheet. Watch video on Atomic structure and Periodic table.

10/15 - Finish Hot Air Balloons. Go over/correct HW. Introduce Periodic Table.
10/14 - Hot Air Balloon Launch.
10/10 - Stamping Science Journals/Hot Air Ballooon Launch. If you know you're going to be absent, please give your journal to a friend who can get it stamped for you. I only stamp journals on Fridays! Thanks.
10/9 - Electron Shells, Valence Electrons, & the Octet Rule. Since there are only 7 possible shells that the electrons can occupy, there is also a very specific order in which each shell fills. You must use the handout with the Electronic Configuration to determine how many electrons are in each shell. If you're interested in finding out the maximum number of electrons that each shell can hold, click on this link:http://education.jlab.org/qa/electron_number.html
For a GREAT interactive periodic table that will show you all the electron configurations, click on the link http://www.chemicalelements.com/show/name.html Once you click on an element, scroll down to see a diagram of the atom with all it's electrons! An example of Uranium with it's 7 electron shells is below.


An atom's Valence refers to how many electrons there are in the last electron shell. For example, Fluorine has a valence of 7 and Neon has a valence of 8.
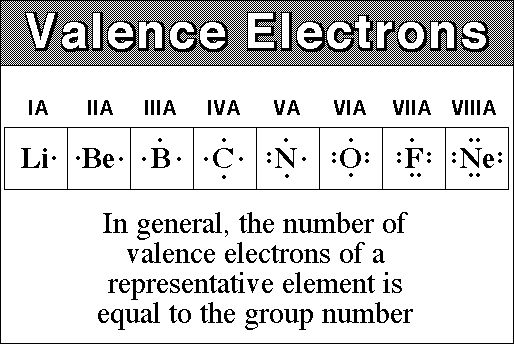
The valence is important because it tells us how an electron will react with other atoms. All atoms want a valence of 8 (they're 'happy" when they have a full outer shell of 8 electrons), and they will lie, cheat, borrow or steal electrons to get 8. This is known as the Octet rule.
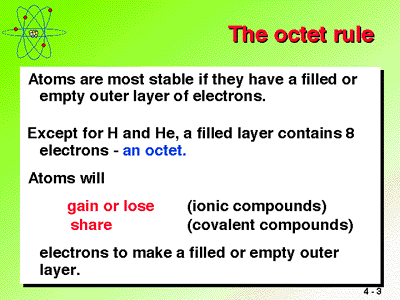
See this link for an explanation/example. http://www.chem4kids.com/files/atom_bonds.html
10/8 - Electron Shell Configuration. The electrons orbits the nucleus in areas called electron clouds. For this class we are going to simplify it and say that electrons orbit in shells around the nucleus. The more energy an electron has, the farther away from the nucleus it will orbit. There are only 7 possible shells and are labeled starting with "K" (the shell closest to the nucleus) moving outward with "Q" being the farthest shell away from. http://www.chem4kids.com/files/atom_orbital.html In the diagram of the Iron Atom below, the inner most shell (K) has 2 electrons, the next shell has 8 electrons, the third shell has 14 and the last shell has only 2 electrons.

More information about the electron configuration of Iron can be found at the link:http://www.chem4kids.com/files/elements/026_shells.html
10/7 - Digital Storytelling. Work with your partner on the voiceover movie script.
10/6 - Ions and Isotopes. Normally an atom has the same number of protons and electrons. For example, Helium has an atomic number of 2, so it has 2 (positively charged) protons and 2 (negatively charged) electrons..giving it an overall neutral (no) charge. If for some reason, the Helium atom gains an extra electron, giving it 3 ..then it would have an overall negative charge, making it a Negative ION. If on the other hand, it lost one of it's electrons, then it would have an overall positive charge, making it a Positive ION!! http://www.chem4kids.com/files/atom_ions.html
An Isotope is when the number of protons and neutrons aren't the same. See the link for a better explanation of Isotopes.http://www.chem4kids.com/files/atom_isotopes.html
Below are examples of the other two isotopes of hydrogen. The Hydrogen atom normally has 1 proton and no neutron. The two isotopes of hydrogen, Deuterium and Tritium are shown below.


Scientists use an isotope of carbon (Carbon-14) to date fossils. See link about how carbon 12 turns into carbon 14..and then decays (changes) into Nitrogen!https://mail.franklinlakes.k12.nj.us/~sschick/004D004D-000F4F25.0/carbon-14.gif
10/3 - Atomic Number and Atomic Mass (Weight) Number. The Atomic number refers to the number of protons in the nucleus. The Atomic Mass or weight is the sum of protons and neutrons in the nucleus. How can you calculate the number of neutrons in the nucleus? The number of electrons always equals the number of protons since they both have to cancel each other out. 
http://www.docbrown.info/page04/4_71atom/Image65.gif
10/2 - Subatomic Particles. http://www.cyberphysics.pwp.blueyonder.co.uk/graphics/diagrams/atomic/atom.gif
The nucleus is so small..and the electrons orbit so far away from the nucleus...the atom is considered to be made up of mostly empty space. If we could enlarge the nucleus to the size of a pea..and put it on the 50 yard line of a football field..the electrons would be orbiting in the stadium with the people.
 http://upload.wikimedia.org/wikipedia/commons/d/dd/Olympic_stadium_football_field.JPG
http://upload.wikimedia.org/wikipedia/commons/d/dd/Olympic_stadium_football_field.JPG
10/1 - Dalton's Atom. John Dalton came up with 4 very important ideas about the atom that still hold true today. 1) all elements are compose of atoms that cannot be divided. 2) all atoms of the same element are exactly alike and have the same mass. 3) atoms cannot change into different atoms, they just rearrange themselves (law of conservation of matter!!) 4) compounds are composed of different atoms combined in specific rations (water H2O)
The basic model of the atom has a nucleus surrounded by electrons. The nucleus contains protons (positive charge) and neutron (neutral = no charge). The electrons orbit far away from the nucleus and have a negative charge. Since opposite charges are attracted to each other (like magnets), the electrons (negative) are held in orbit around the nucleus because of their attraction to the positive protrons. Think of it like the planets in our solar system orbiting around the sun..they are held in their orbits because of the sun's gravitation attraction.

diagram of an atom: http://www.humanthermodynamics.com/Atom_diagram.jpg
9/30 - Journal Collection & grading. Watching an unsolved mysteries video about alien abductions. With so many people around the world claiming they've been abducted by aliens, is it possible that this really happens? There have been many documented sightings of UFOs, and alien abductees claim they are telling the truth..and have even passed lie detector tests. Does this mean it really happened? It just means that they believe they are telling the truth, it doesn't mean it actually happened. You be the judge.....
9/29 - Chapter 3 Test!!
9/26 - Fluids and Solids review: When liquids lose enough energy they become solids; to do this, the molecules that make up the liquid must arrange themselves into a lattice structure. Think of it like this,..when you get to PE class, everyone is crowded together, bunched up in random order, moving pass one another..but then your PE teacher (Mr. Wong) tells you to spread out in order to do warm-up excercises (jumping jacks). When you spread out and get an arms distance between each other, that's when you're forming a lattice..and turning into a solid.
In science liquids and gases are both considered fluids. Huh? Think of it this way..have you ever seen a manta ray swimming underwater? It almost looks like it's gracefully flying through the air. In much the same way, birds fly though the air. Both gases and liquids have similar properties and behave in much the same way..so they're both considered fluids. There is a whole field of science called fluid dynamics where people study
9/25 - Temperature Scales & Viscosity Review: The three temperature scales (see page 26 in textbook). http://courses.nnu.edu/ph106wj/images/temperature_scale.gif
Viscosity is a liquid's resistance to flowing. In other words, the higher the viscosity the slower it moves.
Examples of liquids with high viscosity: honey and maple surup. Temperature is one thing that affects viscosity. The colder a liquid is..the more viscous. The warmer a liquid is, the less viscous it is. Think of maple surup..if you warm it up on the stove...it becomes a lot more runny when you pour it over your waffles or pancakes.
9/24 - Physical vs. Chemical Changes: A physical change is when matter changes state..such as going from a solid to liquid state..(ie. ice melting) In other words, the physical appearance just changes, it's still the same thing. In many ways it's kind of like the robots in the popular movie/tv series "Transformers"..where the robots could change their physical appearance, but they were still the same robot. A Chemical change is when the object changes into something completely different and new. For example, if you take a piece of wood and burn it..it's not the same thing afterwards.. Or if you let a piece of iron rust.. the rust is something completely new and different from what it was before. Fire and color changes are usually good indications that something is undergoing a chemical change. Here's a question for you: If you take a copper penny and melt it using a blow torch...has it undergone a physical or chemical change.. If you said chemical change..think again!..it was copper before..and copper after..so it's physical!
9/23 - The Law of Conservation of Matter : Matter is neither created nor destroyed, it just gets recycled. In other words all the atoms here on Earth have been here ever since the Earth was created about 5 billion years ago..And the atoms that make up your body have been here that long as well. In other words, you're not just 13 or 14 years old, but you're really about 5 billion years old. Ever heard of that saying "you are what you eat".. if you eat a hamburger from McDonalds, ..the beef (or cow) gets broken down by your body..and the reassembled into tissue that you need..such as skin, bone, hair, muscles etc... When you die, your body will go back into the ground, ..and decompose..and other plants and decomposers will absorb and use the nutrients ..and you will become part of the ongoing food chain cycle... gross but true. The matter (atoms) that make up your body will get recycled as well. Your body probably is probably recycled from dinosaurs!
Comments (0)
You don't have permission to comment on this page.